Accelerating Radiopharmaceutical Innovation
BAMF Health is transforming personalized medicine through radiopharmaceuticals, enabling earlier detection, more accurate diagnosis, and targeted treatment for diseases like cancer, Alzheimer’s, and beyond.
To bring this vision to life, we created BAMF RadioNexus: the first-of-its-kind clinical trials network dedicated exclusively to radiopharmaceutical research.

What is BAMF RadioNexus?
BAMF RadioNexus is a specialized clinical trials platform dedicated to radiopharmaceutical innovation. We partner with pharmaceutical sponsors and health systems to overcome the operational, regulatory, and logistical complexities that often slow these critical studies down.
Whether you’re advancing targeted radiopharmaceuticals or expanding your site’s capacity for theranostic research, RadioNexus helps you build infrastructure, grow expertise, and access the support needed to initiate and complete complex trials faster and with confidence.
Why Partner with BAMF RadioNexus?
For Pharmaceutical Sponsors
- Faster, more reliable study startup and execution
- Access to diverse patient populations
- Consistent trial workflows and high-quality data capture
- Experienced, high-performing partners
For Clinical Sites and Health Systems
- Tailored onboarding and training
- Best practices in regulatory navigation and operational readiness
- Priority access to cutting-edge trials
- Grow research capabilities and patient care offerings
Our Process
We analyze your site’s current clinical research capabilities and develop a customized roadmap for launching and scaling clinical trials across single or multi-location systems.
We equip your site with operational infrastructure, including scheduling workflows, imaging and data capture processes, radiation safety tools, budgeting templates, team training, and more.
We assist with site qualification and feasibility, regulatory submissions and IRB support, development of protocol-specific workflows, and site onboarding.
We identify and act on opportunities to improve enrollment, reduce protocol deviations, increase cross-site coordination, and drive operational efficiencies.
Ready to Shape the Future of Personalized Care?
We are currently enrolling patients for the following advanced clinical trials. If you'd like more information about which trial is best for you, complete this general interest form.
Recurrent Brain Metastases from Solid Tumors | RAD101 Imaging Copy link An Open-Label, Single Dose, Single Arm, Multicenter Phase 2b Study to Establish the Imaging Performance of RAD101 Positron Emission Tomography (PET) in Participants with Suspected Recurrent Brain Metastases from Solid Tumors (GCP-PRT-101-001).
Protocol Title: An Open-Label, Single Dose, Single Arm, Multicenter Phase 2b Study to Establish the Imaging Performance of RAD101 Positron Emission Tomography (PET) in Participants with Suspected Recurrent Brain Metastases from Solid Tumors (GCP-PRT-101-001)
Sponsor: Radiopharm Theranostics Ltd.
Description: Contact BAMF for more information
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): N/A
Patient Eligibility Criteria: Contact BAMF for more information
If you are interested in more information about this trial, please fill out this form.
Pancreatic, Non-Small Cell Lung, Breast, and Colorectal | Novartis Imaging Therapy Copy link The purpose of this study is to evaluate the safety, tolerability, dosimetry and preliminary efficacy of [177Lu]Lu-NNS309 and the safety and imaging properties of [68Ga]Ga-NNS309.
Protocol Title: Phase I Open-label, Multi-center Study to Evaluate the Safety, Tolerability, Dosimetry, and Preliminary Activity of [177Lu]Lu-NNS309 in Patients With Pancreatic, Lung, Breast and Colorectal Cancers
Sponsor: Novartis Pharmaceuticals
Description: The purpose of this study is to evaluate the safety, tolerability, dosimetry and preliminary efficacy of [177Lu]Lu-NNS309 and the safety and imaging properties of [68Ga]Ga-NNS309 in patients aged ≥ 18 years with locally advanced or metastatic pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), HR+/HER2- ductal and lobular breast cancer (BC), triple negative breast cancer (TNBC) and colorectal cancer (CRC).
The study will be done in two parts. The first part is called “escalation” and the second part is called “expansion”. In both parts of the study, patients will initially be imaged with a [68Ga]Ga-NNS309 positron emission tomography (PET)/ computed tomography (CT) or PET/magnetic resonance imaging (MRI) scan and will be evaluated for eligibility for [177Lu]Lu-NNS309 treatment. In the escalation part, different doses of [177Lu]Lu-NNS309 will then be tested to identify recommended dose(s) (RD(s)) for further evaluation. The expansion part of the study will examine the safety and preliminary efficacy of [177Lu]Lu-NNS309 at the RD(s) determined during the escalation part. The end of study will occur when at least 80% of the patients per disease group in the expansion part have completed the follow-up for disease progression or discontinued from the study for any reason, and all patients have completed treatment and the 36-month long-term follow-up period.
Patient Enrollment at BAMF: Early November 2024
Number of patients to be enrolled (study wide): 124
Patient Eligibility Criteria: please click here to see the complete list at ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Urothelial Carcinoma | Aktis Nectinium 2 Therapy Copy link A Phase 1b, 2-part, Multicenter, Single-arm, Open-label Study to Evaluate the Safety and Efficacy of a Nectin-4 Radiopharmaceutical ([225Ac]Ac-AKY-1189) in Patients with Previously Treated Locally Advanced or Metastatic Solid Tumors
Protocol Title: NECTINIUM-2: A Phase 1b, 2-part, Multicenter, Single-arm, Open-label Study to Evaluate the Safety and Efficacy of a Nectin-4 Radiopharmaceutical ([225Ac]Ac-AKY-1189) in Patients with Previously Treated Locally Advanced or Metastatic Solid Tumors
Sponsor: Aktis Oncology
Description: This is a phase 1b, 2-part, multi-center, single-arm, open-label study to evaluate the safety, tolerability, dosimetry, and pharmacokinetics (PK) of [225Ac]Ac-AKY-1189 and/or [64Cu]Cu-AKY-1189 as well as the preliminary anti-tumor activity of [225Ac]Ac-AKY-1189 in patients with locally advanced or metastatic urothelial cancer (mUC) and Nectin-4-expressing locally advanced or metastatic solid tumors. The study will consist of two parts.
Part 1 dose escalation includes patients with metastatic urothelial cancer (mUC).
Part 2 dose expansion includes patients with Metastatic Triple Negative Breast Cancer, HR+ Breast, Non-Small Cell Lung, Cervical, Colorectal, and Head & Neck cancers
The Part 1 dose escalation portion will determine the maximum tolerated or maximum administered dose (MAD) and the recommended Part 2 dose (RP2D) of [225Ac]Ac-AKY-1189 in ~30 patients with locally advanced or metastatic urothelial cancer. This will be followed by a dose expansion in Part 2 to evaluate the efficacy and safety of [225Ac]Ac-AKY-1189 at the RP2D in 3 cohorts of patients as described below.
BAMF Health is currently enrolling to Part 1 for all disease sites.
Patients Enrollment at BAMF: ~150
Number of patients to be enrolled (study wide): Approximately 150 total
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Inoperable or Metastatic Solid Tumors | RAD BetaBart Therapy Copy link A Phase 1/2a Dose Escalation and Expansion Study of the Safety, Tolerability, and Preliminary Clinical Activity of 177LuBetaBart, a 177Lu-Labeled Anti-B7-H3 Monoclonal Antibody, in Patients with Relapsed/Refractory, Locally Advanced Inoperable, or Metastatic Solid Tumors
Protocol Title: A Phase 1/2a Dose Escalation and Expansion Study of the Safety, Tolerability, and Preliminary Clinical Activity of 177LuBetaBart, a 177Lu-Labeled Anti-B7-H3 Monoclonal Antibody, in Patients with Relapsed/Refractory, Locally Advanced Inoperable, or Metastatic Solid Tumors
Sponsor: Radiopharm Theranostics Ltd. (RAD)
Description: The purpose of this study is to establish the safety profile, biodistribution, pharmacokinetics (pk), and radiation dosimetry of 177Lu-BetaBart, to determine the maximum tolerated dose (MTD) and/or recommended Phase 2 dose (RP2D), and to evaluate preliminary anti-tumor activity in select patient populations.
The study is divided into 2 phases. Phase 1 is the dose escalation phase. Phase 2a is the dose expansion phase. Each phase consists of a Screening Period, a Treatment and Imaging Period, and a Safety and Long-term Follow-up Period.
Trial population: Participants ≥ 18 years of age with castration-resistant prostate cancer (CRPC), colorectal cancer (CRC), non-small-cell lung cancer (NSCLC), small-cell lung cancer (SCLC), head and neck squamous cell carcinoma (HNSCC), ovarian cancer, cervical cancer, endometrial cancer, triple negative breast cancer (TNBC), or esophageal squamous cell carcinoma (ESCC) who have documented disease progression during or after their most recent line of anticancer therapy will be eligible to enroll. CRC will be capped at 33% of enrollment per cohort.
Patient Enrollment at BAMF: Trial will be active and enrolling in November 2025
Number of patients to be enrolled (study wide): 61
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.Gov
If you are interested in more information about this trial, please fill out this form.
GRPR Solid Tumors | Eli Lilly OMNIRAY Therapy Copy link A Study to Investigate LY4257496 in Adults with GRPR-Positive Advanced Solid Tumors. Phase: 1a/1b
Protocol Title: A Study to Investigate LY4257496 in Adults with GRPR-Positive Advanced Solid Tumors. Phase: 1a/1b
Sponsor: Eli Lilly and Company
Description: This is a Phase 1a/b multicenter, open-label trial of LY4257496, a GRPR-targeted radioligand therapy, in adults with GRPR-positive advanced solid tumors (OMNIRAY). The trial will evaluate safety, tolerability, and efficacy of LY4257496 as monotherapy and as part of relevant SOC combination therapy in participants with GRPR-positive advanced solid tumor types, including Breast, Colorectal, Prostate and Endometrial cancer.
GRPR-positive disease will be defined by GRPR imaging at screening.
Brief Summary: Phase 1a will enroll up to 135 participants, Phase 1b will enroll up to 286 participants, both phases will include : Breast, Colorectal, Prostate and Endometrial cancer.
Patient Enrollment at BAMF: Mid-November 2025
Number of patients to be enrolled (study-wide): 421 Patients.
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.Gov
If you are interested in more information about this trial, please fill out this form.
FAP-Expressing Solid Tumors | Eli Lilly FIREBOLT Therapy Copy link Protocol Title: A Dose Escalation and Dose Optimization Phase 1a/1b Study to Evaluate Safety, Tolerability and Dosimetry of Radioligand Therapy With LY4337713 in Adults With FAP-Positive Solid Tumors (FiREBOLT)
Protocol Title: A Dose Escalation and Dose Optimization Phase 1a/1b Study to Evaluate Safety, Tolerability and Dosimetry of Radioligand Therapy With LY4337713 in Adults With FAP-Positive Solid Tumors (FiREBOLT)
Sponsor: Eli Lilly and Company
Description: This is a study of LY4337713 in participants with certain types of cancer that is advanced or has spread. Participants must have cancer with high levels of a protein called fibroblast activation protein (FAP). The purpose of this study is to evaluate safety, side effects, and efficacy of LY4337713. In addition, this study will evaluate how much LY4337713 gets into the bloodstream, how it is broken down, and how long it takes the body to get rid of it. For each participant, the study will last about 5 years. The trial will enroll patients with ovarian, breast, pancreatic, colorectal, esophageal, stomach, and cholangiocarcinoma cancers.
Patient Enrollment at BAMF: Late January of 2026
Number of patients to be enrolled (study wide): 241
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form
Endometriosis | BAMF Health Imaging Copy link This is a single-site exploratory research study, not a clinical trial, aimed at providing an initial understanding of the diagnostic potential of [18F]FAPI-74 PET-MRI scans for identifying ectopic endometrial lesions.
Protocol Title: Imaging and Biodistribution of [18F]FAPI-74 in Endometriosis Using PET/MRI
Sponsor: BAMF Health
Description:
This is a single-site exploratory research study, not a clinical trial, aimed at providing an initial understanding of the diagnostic potential of [18F]FAPI-74 PET-MRI scans for identifying ectopic endometrial lesions. The study will include up to 5 women with suspected endometriosis, aged 18-45, who are already scheduled for surgery to confirm the diagnosis. Participants will undergo one PET/MRI imaging session at BAMF Health before surgery. This imaging approach aims to identify and localize endometriotic lesions more accurately than current diagnostic methods.
Screening assessments will confirm eligibility before enrollment. Participants will receive a single injection of [18F]FAPI-74 before the PET/MRI scan. A follow-up phone call will occur 24-72 hours after the imaging session to monitor for any side effects.
Patient Enrollment at BAMF: Recruiting now
Number of patients to be enrolled (study wide): 10
Patient Eligibility Criteria: view patient eligibility criteria here
If you are interested in more information about this trial, please fill out this form.
Liver Cancer | RayzeBio, RYZ801-101 Imaging Therapy Copy link A single arm, open-label Phase 1/1b study of the theranostic pair RYZ811 (diagnostic) and RYZ801 (therapeutic) to identify and treat subjects with GPC3+ unresectable HCC.
Protocol Title: A single arm, open-label Phase 1/1b study of the theranostic pair RYZ811 (diagnostic) and RYZ801 (therapeutic) to identify and treat subjects with GPC3+ unresectable HCC
Sponsor: RayzeBio, Inc.
Description: To determine the recommended phase 2 dose (RP2D) of RYZ801 in subjects with GPC3+ unresectable HCC (Dose Escalation only). To assess the safety and tolerability of RYZ801 in subjects with GPC3+ unresectable HCC. Safety and tolerability of RYZ801 as measured by incidence and severity of AEs, including SAEs, laboratory changes, and other safety findings. To assess the safety and tolerability of RYZ811 in subjects with unresectable HCC. Safety and tolerability of RYZ811 as measured by incidence and severity of AEs, including SAEs, laboratory changes, and other safety findings. To evaluate the biodistribution of RYZ811 in subjects with unresectable HCC. SUV (mean, maximum and peak) of organs and tumors; volume of RYZ811 avid uptake in tumor lesions; and tumor-to-normal organ tracer uptake ratios.
Key Inclusion Criteria:
Age of at least 18 years at the time of signing the informed consent form (ICF)
Histologically/cytologically confirmed diagnosis of HCC.
At least 1 prior systemic therapy for unresectable HCC
Key Exclusion Criteria:
Subjects with fibrolamellar carcinoma, sarcomatoid HCC or combined hepatocellular cholangiocarcinoma
Prior liver transplantation or candidates for liver transplantation
Documented hepatic encephalopathy
Prior EBRT to the liver
Prior liver radioembolization
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): ~70
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Neuroendocrine Tumors | Perspective Therapeutics Therapy Copy link Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors
Title: A Phase I/IIa First-in-Human Study of [212Pb]VMT-α-NET Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors
Sponsor: Perspective Therapeutics
Description: This is a prospective, multi-center open-label dose escalation, dose expansion study of [212Pb]VMT-α-NET in up to 160 adult subjects with unresectable or metastatic SSTR2-expressing neuroendocrine tumors (NETs) who have not received prior peptide receptor radionuclide therapy (PRRT).
The radioactivity dose escalation period (Phase I) tests up to 4 escalating radioactivity dose cohorts of up to 8 subjects (administered at approximately 8-week intervals) at the assigned cohort radioactivity dose.
Pre-specified dose adjustments and individual stopping rules for repeat treatment cycles are based on observed dose-limiting toxicities (DLTs) and adverse events (AEs).
Additionally, up to 40 subjects may be enrolled in each of the cohorts.
The Maximum Tolerated Dose (MTD) will be determined based on observed DLTs within 42 days of the first treatment cycle.
The recommended expansion (Phase IIa) dose(s) will be determined following a holistic analysis of observed DLTs, AEs, estimated cumulative organ radiation exposure, and efficacy signals over the course of all treatment cycles for all dose cohorts.
If MTD can not be identified within the 4 radioactivity dose cohorts, a Maximum Feasible Dose (MFD), incorporating manufacturing and logistical considerations for [212Pb]VMT-α-NET production, may be determined.
Up to 120 subjects will be considered for enrollment in the dose-expansion phase (Phase IIa) with approximately 100 subjects with GEP-NETs, approximately 10
subjects with bronchial NETs [small cell lung cancer], and approximately 10 subjects with pheochromocytoma or paragangliomas)
Reno-protective amino acids will be co-administered in a separate IV line prior to each [212Pb]VMT-α-NET dose in all subjects. Escalation will be based on a modified toxicity probability interval design [mTPI-2] until MTD is identified or the pre-specified rules are met.
A lead-in dosimetry sub-study will be conducted during the dose escalation period in which all subjects in the first two dose cohorts will undergo dosimetric evaluation prior to receiving the therapeutic agent.
Patient Enrollment at BAMF: recruiting now
Number of patients to be enrolled (study wide): 280
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Prostate Cancer Imaging | Archeus ART-101 Imaging Copy link A Phase 1 Safety and Feasibility Study of [111In]In-ART-101 in Men with Metastatic Castrate-resistant Prostate Cancer
Protocol Title: A Phase 1 Safety and Feasibility Study of [111In]In-ART-101 in Men with Metastatic Castrate-resistant Prostate Cancer
Sponsor: Archeus Technologies
Description: This is a phase 1 safety and feasibility SPECT/CT imaging study of [111In]In-ART-101 in approximately 10 participants with mCRPC who are candidates to receive radioligand therapy with [177Lu]Lu-PSMA-617 (Pluvicto). While there is no anticipated clinical benefit to participants enrolled in this study, verification in human participants of the biodistribution characteristics of ART- 101 observed in preclinical studies could result new imaging or therapeutic agents, which could potentially benefit future patients.
Each patient will undergo a total of eight SPECT/CT scans, with four scans after [111In]In-ART-101 administration and four scans after [177Lu]Lu-PSMA-617. Participation in the study will last approximately two months across the screening, study treatment, and follow-up periods.
Patient Enrollment at BAMF: Mid-June 2025
Number of patients to be enrolled: ~10
Patient Eligibility Criteria:
-Patient must be diagnosed with metastatic castration-resistant prostate cancer and be a candidate to receive radioligand therapy with [177Lu]Lu-PSMA-617.
-Patient must have had a PSMA-positive PET/CT within 8 weeks prior to being enrolled with scans available.
-Patient must not be undergoing other concurrent cytotoxic chemotherapy, immunotherapy, radiopharmaceutical therapy, or investigational therapy
If you are interested in more information about this trial, please fill out this form.
Prostate Cancer Imaging | BAMF Health ULTRAgLOW Imaging Copy link Minimizing CT Radiation in Total-Body PET-CT: A Head-to-Head Comparison of Low- Vs Standard-Dose CT for PSMA Imaging in Prostate Cancer
Protocol Title: Minimizing CT Radiation in Total-Body PET-CT: A Head-to-Head Comparison of Low- Vs Standard-Dose CT for PSMA Imaging in Prostate Cancer
Sponsor: BAMF Health
Description: In this pilot study, BAMF Health is using the United Imaging uEXPLORER total-body PET-CT scanner along with AI Image Reconstruction (AIIR) software. The goal is to test new protocols that can lower radiation exposure from the CT portion of a scan to 22% or less, compared to traditional PET-CT scanners.
The integration of AIIR for CT dose reduction presents a pioneering approach to better understand a patient’s disease and enhance patient safety, particularly for those requiring frequent scans, such as patients with chronic conditions or cancer, thereby potentially reshaping diagnostic standards in healthcare
As a part of their standard of care imaging, patients in this study will receive the ordered PET tracer through an IV, following BAMF Health’s strict clinical policies. After the injection, a whole-body PET-CT scan will be performed using both the standard and reduced radiation doses.
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): ~30
Patient Eligibility Criteria: To be eligible to participate in this study, an individual must meet all the following criteria:
- Provision of signed and dated informed consent form
- Stated willingness to comply with all study procedures and availability for the duration of the study
- Age ≥ 18 years of age
- Referred to BAMF Health for PSMA PET-CT imaging for standard medical care
- No serious co-morbidities, serious disease, severe claustrophobia or pain that in the opinion of the investigators, and/or physician of record could compromise participant safety, protocol objectives, and/or ability to remain still in supine position for duration of imaging.
If you are interested in more information about this trial, please fill out this form.
Prostate Cancer Therapy | ARTBIO Artisan Therapy Copy link An Open-label, Multicenter Phase 1/2 Study to Investigate the Safety, Tolerability, Pharmacokinetics, Biodistribution and Antitumor Activity of the Alpha Radioligand Therapy AB001 in Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC)
Protocol Title: An Open-label, Multicenter Phase 1/2 Study to Investigate the Safety, Tolerability, Pharmacokinetics, Biodistribution and Antitumor Activity of the Alpha Radioligand Therapy AB001 in Patients with Metastatic Castration Resistant Prostate Cancer (mCRPC)
Sponsor: ARTBIO
Description: This is a Phase 1/2 dose escalation study to investigate if participants with advanced mCRPC can be treated with AB001 with an acceptable safety profile and expected positive benefit/risk ratio, and to obtain preliminary data on antitumor activity.
The dose escalation design will allow determination of the recommended dose and treatment schedule of AB001, for further evaluation and refinement during the dose expansion part of the trial. Furthermore, evaluation will be undertaken of the biodistribution, pharmacokinetics (PK), and body clearance for AB001 to support development of this ART.
Dose Escalation: The number of participants treated is estimated to be approximately 30.
Dose Expansion: Two separate Groups (A and B) of 30 participants each will be treated.
Patient Enrollment at BAMF: actively enrolling
Number of patients to be enrolled (study wide): 90
Patient Eligibility Criteria: Contact BAMF Health for more information
If you are interested in more information about this trial, please fill out this form.
Prostate Cancer Therapy | Clarity SECuRE Imaging Therapy Copy link A Phase I/IIa theranostic study of 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for identification and treatment of PSMA-expressing metastatic castrate resistant prostate cancer.
Protocol Title: A Phase I/IIa theranostic study of 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for identification and treatment of PSMA-expressing metastatic castrate resistant prostate cancer.
Sponsor: Clarity Pharmaceuticals Ltd.
Description: This is a multi-center, open-label, non-randomized, dose-escalation, dose finding, cohort expansion study of 64Cu-SAR-bisPSMA and 67Cu-SARbisPSMA administered to participants with metastatic castrate resistant prostate cancer (mCRPC).
BAMF Health will be enrolling into both groups of the Cohort Expansion Phase: Main and Enzalutamide
Patient Enrollment at BAMF: Trial will be active & enrolling October 2025
Number of patients to be enrolled (study wide): 24
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.Gov
If you are interested in more information about this trial, please fill out this form.
Metastatic Sarcoma | Lantheus FAPI-1301 Imaging Copy link This is a prospective Phase 1/2a study to assess safety and tolerability of 64Cu-LNTH-1363S (64Cu Radiolabeled FAPi PET/CT Imaging Agent).
This is a multicenter, open-label, prospective Phase 1/2a study to assess safety and tolerability, establish dosimetry and to identify an optimal imaging dose (radioactivity and mass dose) and imaging time window of 64Cu-LNTH-1363S (64Cu Radiolabeled FAPi PET/CT Imaging Agent) and to compare its imaging biodistribution with FAP expression by immunohistochemistry (IHC) in patients with sarcomas or GIT cancers. The study will be conducted in 2 parts (Part 1 and Part 2).
Part 1 will include 12 evaluable patients with supposed FAP-expressing solid tumors (metastatic sarcomas).
Part 1 of the study will last approximately 3 weeks for each patient and includes a Screening Period (up to 14 days), a 1-day Intervention Period, and a Safety Follow-up Period (7 days post dose).
BAMF Health is currently only enrolling to Part 1 (Metastatic Sarcoma)
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): ~32 Patients study wide
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.Gov
If you are interested in more information about this trial, please fill out this form.
Small Cell Lung Cancer | RYZ101-101 Therapy Copy link Small Cell Lung Cancer | RYZ101-101
Protocol Title: Study of RYZ101 in Combination With SoC in Subjects With SSTR+ ES-SCLC
Sponsor: RayzeBio, Inc.
Description: This Phase 1b study aims to determine the safety, preliminary antitumor activity, and pharmacokinetics (PK) of RYZ101 in combination with standard of care (SoC) therapy consisting of carboplatin + etoposide + atezolizumab in untreated subjects with somatostatin receptor expressing (SSTR+) ES-SCLC.
Patient Enrollment at BAMF*: Open to enrollment
Number of patients to be enrolled (study wide): 31
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
*This trial is conducted in collaboration with Corewell Health™. If you complete the interest form, we may provide your information to their staff
BAMF Health Clinical Trial Articles
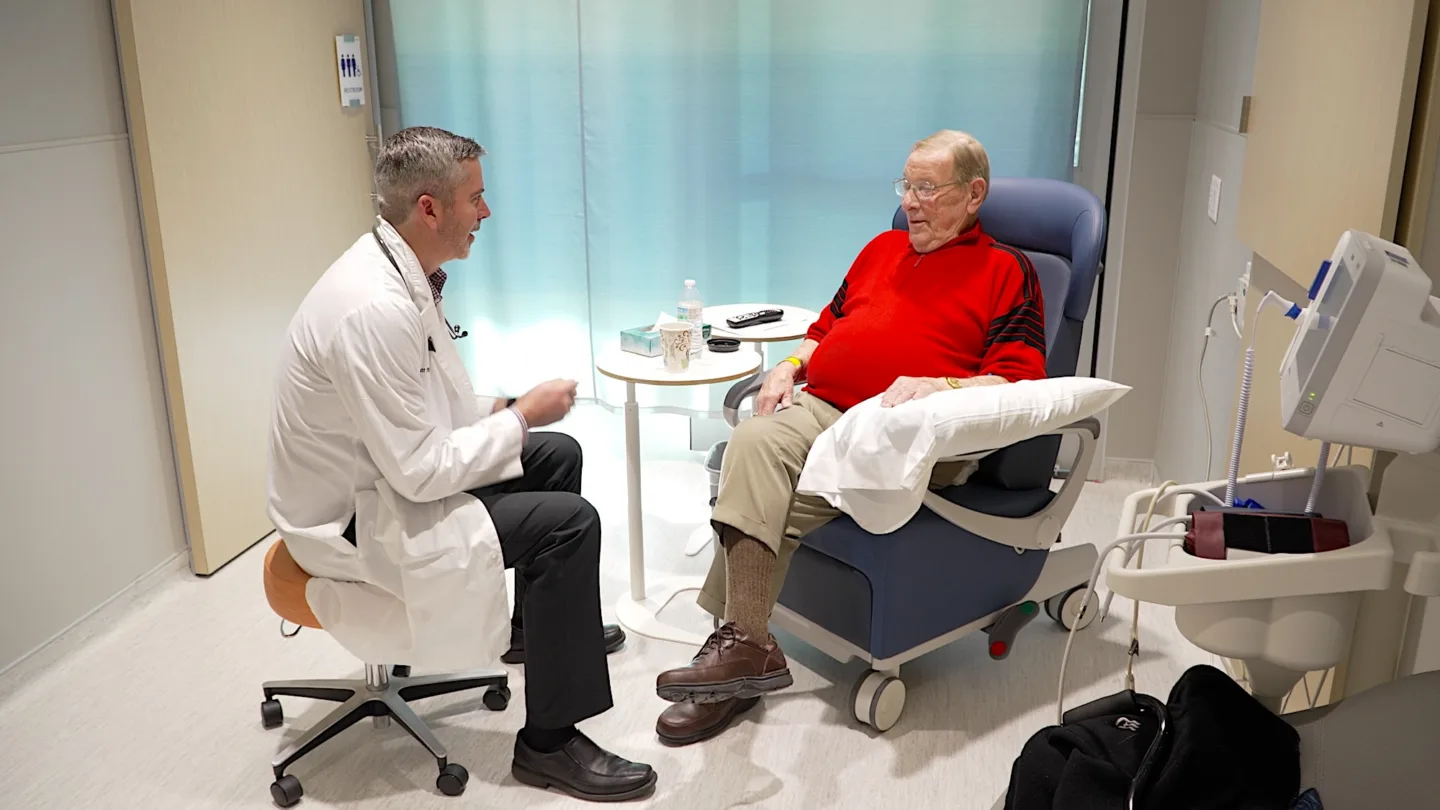
World Traveler Becomes First-in-World Patient
Bill was the first patient in the world to participate in a clinical trial exploring an experimental radiopharmaceutical therapy for prostate cancer.
January 26, 2026

BAMF Health Doses First Patient in Promising New Prostate Cancer Alpha Radioligand Therapy Clinical Trial
BAMF Health dosed the first patient enrolled in a Phase 1 prostate cancer therapy clinical trial sponsored by ARTBIO.
November 25, 2025

It Takes Courage. God Gave Me Courage.
With a zest and appreciation for life, at 77 years old, Barbara considered herself the picture of perfect health. But that changed in June of 2025 when she was diagnosed with metastatic HR-/HER2+ breast cancer.
October 2, 2025