Born in Louisville, Kentucky, 85-year-old U.S. Air Force veteran John Conway calls himself “a Southerner by birth and a Yankee at heart.” Over the years, military service and work carried him and his family around the world: Korea, Mississippi, Ohio, the Carolinas, Texas, Illinois, each place adding a chapter to a life shaped by commitment.


Eventually, that same commitment drew John and his late wife, Eileen, to West Michigan. Being closer to children, grandchildren, and now great-grandchildren mattered more than geography ever had. What John couldn’t have known at the time was that this move would later place him at the center of a historic moment.
A Chance to be the “First”
John was first diagnosed with prostate cancer in the early 2000s. After undergoing external beam radiation, life returned to a familiar rhythm. For nearly two decades, cancer faded into the background. But in 2023, routine bloodwork told a different story. His PSA was rising again.
Conversations with his oncologist led him to BAMF Health, where he learned about an opportunity to be the first patient in the world to participate in a clinical trial evaluating an investigational radiopharmaceutical therapy: the ARTISAN study. John understood there were standard treatment options available to him, but he was also open to learning more, asking questions, listening carefully, and weighing his choices with the same thoughtfulness that had guided much of his life.

BAMF Health Medical Director and Principal Investigator for the trial, Dr. Brandon Mancini, said, “The clinical trial John’s participating in has the potential to reshape the treatment landscape for advanced prostate cancer and bring a new option to patients with metastatic castration-resistant prostate cancer (mCRPC) who urgently need additional pathways forward.”
In this trial, patients receive a radiopharmaceutical called AB001 that delivers alpha-emitting radiation directly to cancer cells. Alpha radiation can cause more damage to cancer cells while limiting harm to nearby healthy tissue.
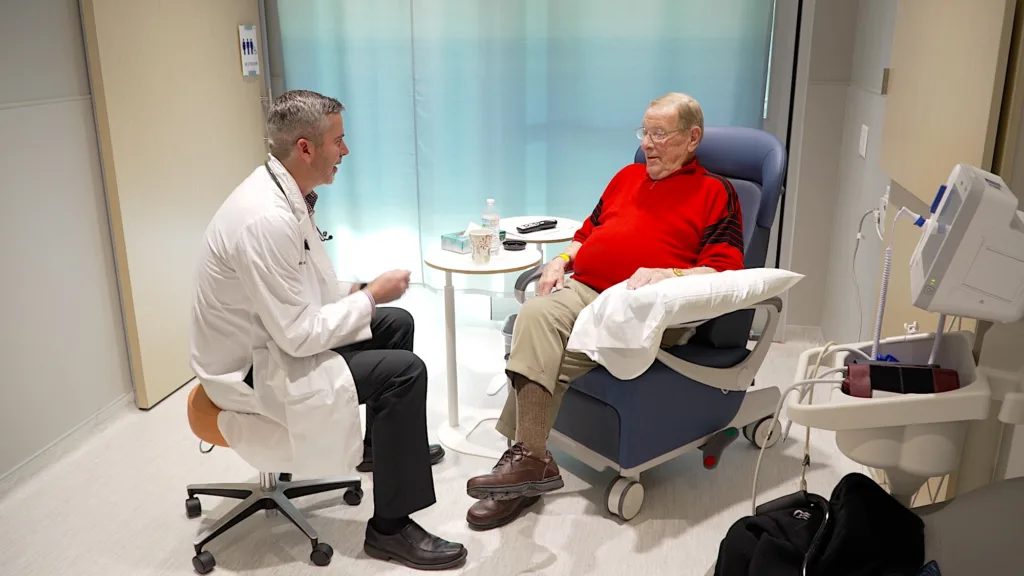
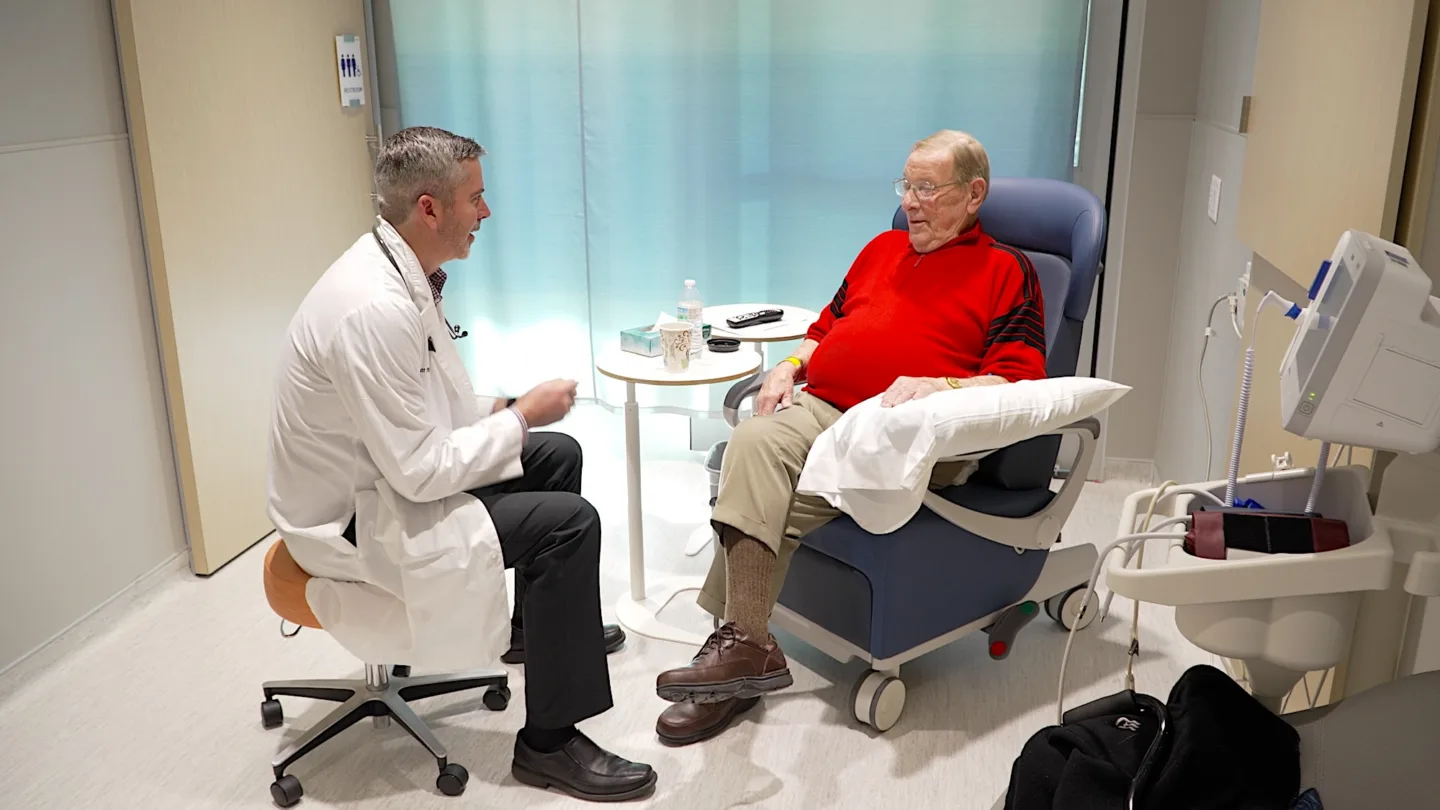
“The BAMF team has been a lot of fun, really,” John said with a smile. “They’re very friendly and jovial. I have no complaints. The facility is new, the setup is comfortable, and the bathroom is private.”
Being Close to Family is What Matters Most
For John, the decision to participate in the trial wasn’t about being the first patient dosed. It was about hope. Hope that his cancer could fade into the background once again so he could continue focusing his time and attention on his family.

“We’re looking forward to seeing how John and our other trial patients respond to this new therapy,” said Dr. Mancini. “Right now, patients have limited options for mCRPC, so we’re excited about the possibility of offering patients multiple options to further personalize treatment for their specific cancer.”
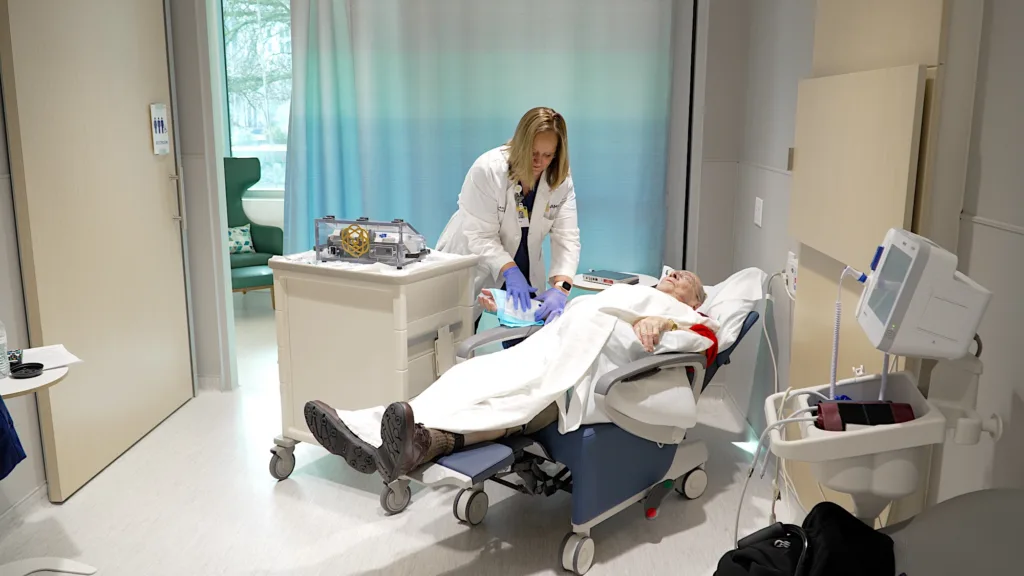
The ability for John to receive such advanced care so close to home means he has more time to spend with family—time with his partner Mary, time listening to grandchildren laugh, time continuing a life that has already been rich with meaning.

“My family is very close and very loving. We’re together on all of the holidays, and we see each other for cookouts in the summer. We’re together pretty frequently, and that’s the reason we moved here.”


