For years, men with metastatic castration-resistant prostate cancer have followed a fairly predictable treatment path. After hormone therapy stopped working, chemotherapy was often the next step. While chemotherapy has been effective for many, it comes with well-known challenges like hair loss, fatigue, nausea, lowered blood counts, and changes in quality of life.
However, thanks to an expanded FDA approval, Pluvicto® is now available earlier in the treatment journey. This radiopharmaceutical therapy targets prostate cancer cells directly, delivering radiation from the inside out. And as of March 2025, patients may be able to receive Pluvicto® before chemotherapy.
At BAMF Health, we’ve been among the first centers in the country to treat patients in this new “pre-chemo” setting. Here’s what we’re learning—and what we’re still working to understand.
Why This Matters for Patients
Pluvicto® represents something patients and physicians have long been hoping for: a treatment as powerful as chemotherapy, but with far fewer side effects.
“Pluvicto® allows patients to have a very targeted, aggressive treatment, but typically without the same side effect burden,” said BAMF Health Medical Director Dr. Brandon Mancini. “That means patients have an opportunity to improve their cancer outcomes while still enjoying day-to-day life—spending time with family, traveling, and doing what they love.”
For many men, this is life-changing. Patients like Mark and Michael, two of BAMF’s first pre-chemo patients, have reported major improvements in their PSA levels, clear evidence of cancer regression on scans, and, most importantly, excellent quality of life.
“I’m on my second treatment and I’ve had little to no side effects other than a little bit of dry mouth and some fatigue,” Michael shared. “But I’ll take that any day. The medicine is doing what it’s supposed to do.”
Early Results: Strong Responses and Better Quality of Life
In the first few months since FDA approval, BAMF Health has observed several encouraging patterns:
- Faster PSA declines: Many patients are seeing quicker drops in PSA, a key marker of prostate cancer activity.
- Visible improvements on scans: SPECT/CT imaging after each treatment shows tumors shrinking or disappearing altogether.
- Improved quality of life: Unlike chemotherapy, patients are often able to continue their usual activities without major side effects.
Dr. Mancini noted that in some cases, patients’ cancers have responded so well that additional treatment doses could be safely delayed. In Michael’s case, he was able to pause therapy after his second treatment cycle.
“Sometimes after just two or three treatments, we see such a robust response that we can pause further cycles of therapy,” Dr. Mancini adds. “That means fewer side effects, lower costs, and more time living life fully without sacrificing effectiveness.”
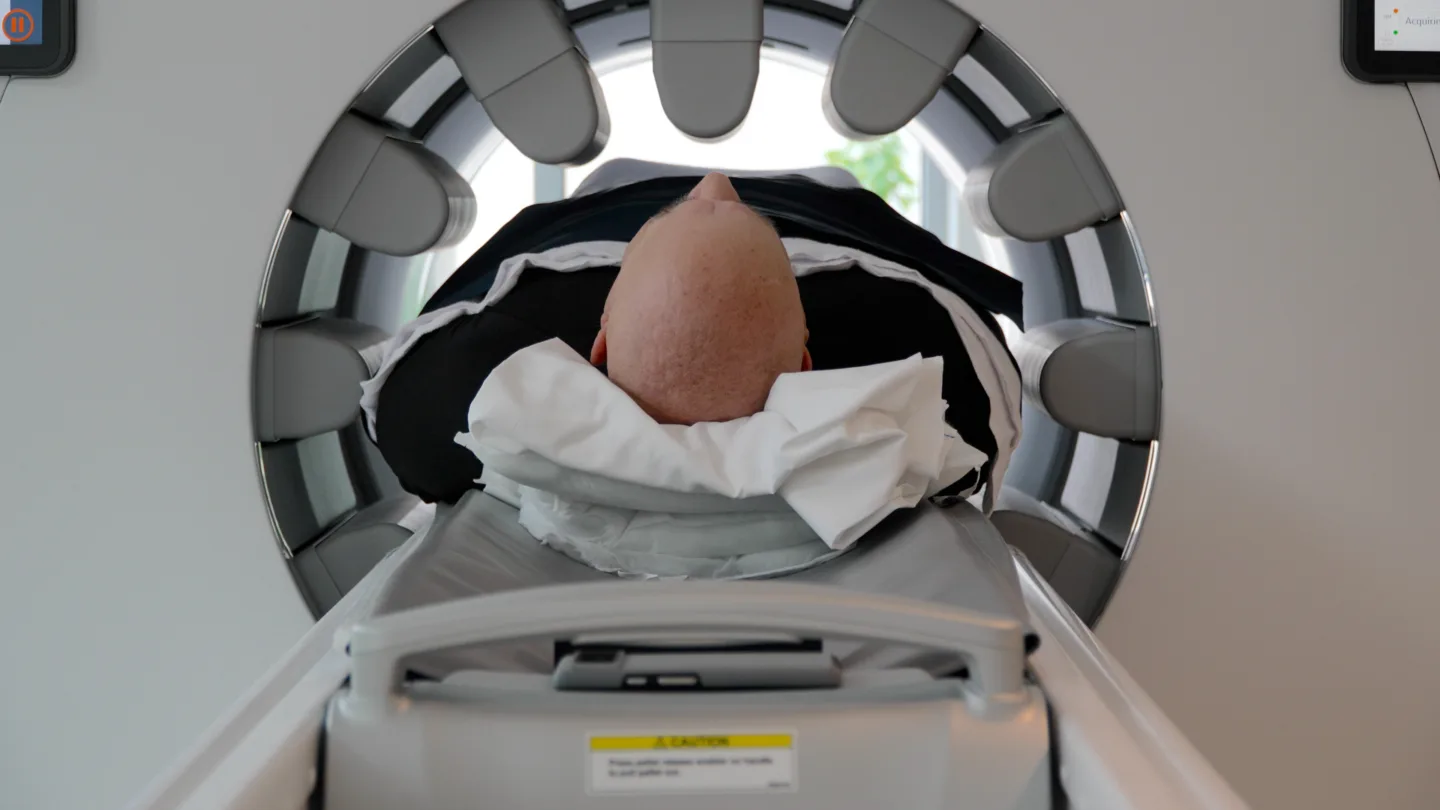
Understanding the Role of SPECT/CT Imaging
One unique aspect of BAMF Health’s approach is the use of SPECT/CT imaging 24 hours after every Pluvicto® treatment. These scans show us exactly how much of the medicine is reaching cancer cells and whether it’s working as intended.
“They show me where the medicine is and how it’s interacting. It’s mind-blowing,” Michael describes. “This is just lightyears ahead. It’s like being on the Starship Enterprise! You see the magic on the screen the next day, and that’s when you know things are really happening and the miracle of this medication.”
For patients, this means care can be more personalized. If the scans show little cancer left for the drug to target, we may recommend taking a break from treatment—preserving future cycles for when they’re truly needed.
Dr. Mancini adds, “It’s about extending the usefulness of Pluvicto® over the longest possible time, while avoiding unnecessary side effects. For patients, that translates to longer, healthier lives.”
What Patients Are Saying
Patients often describe relief when learning they can avoid or delay chemotherapy. Many have seen loved ones go through chemo and fear the side effects. Pluvicto® offers hope—not only for better cancer control but also for more normalcy in everyday life.
“When I heard that they waived the chemo option, I did the happy dance!” Michael said. “I was like, that’s great news.”
“I was looking at more of a targeted approach. So, I wasn’t actively pursuing chemo,” said Mark. “I think I made it known to my doctor that I didn’t like chemo.”
Throughout his treatment, Mark has been able to continue doing all the activities he loves, like biking, running, fishing, and golfing. “Probably wouldn’t be doing that if I were on chemo. I’d probably be sitting at home a lot more often. It makes me feel good.”
What We Still Need to Learn
While early results are very encouraging, we are still gathering long-term data. Some of the key questions include:
- Durability of response: How long will these strong early responses last?
- Optimal sequencing: Should Pluvicto® always come before chemotherapy, or in certain cases is the reverse better?
- Combination therapies: Could pairing Pluvicto® with other treatments (like hormone therapy or targeted drugs) improve results further?
- Genetic and genomic factors: Can certain patient profiles predict who will benefit most?
“Moving Pluvicto® earlier in treatment is a big step forward,” Dr. Mancini said. “But we’re still learning how to best integrate it, what the right order of therapies is, when to combine it, and how to personalize care so every patient gets the maximum benefit.”
Looking Ahead: A Brighter Future for Prostate Cancer Care
Pluvicto® is just the beginning. Clinical trials are already exploring its use earlier in prostate cancer, potentially even at the time of initial diagnosis. Researchers are also investigating next-generation radiopharmaceuticals that may be even more precise, more powerful, and longer-lasting.
At BAMF Health, we believe Theranostics—using imaging and therapy together to target cancer—is transforming cancer care as we know it. As Dr. Mancini put it:
“Theranostics is here to stay. The science keeps evolving, and people will live longer, healthier lives because of it. Pluvicto® moving before chemotherapy is proof of that progress.”
For men with metastatic prostate cancer, the FDA’s decision to allow Pluvicto® before chemotherapy has opened an important new pathway. It means more choices, more hope, and more time spent living life rather than managing side effects.
At BAMF Health, we’re proud to be at the forefront of this change. We are learning alongside our patients, tracking outcomes carefully, and continually working with our medical oncology and urology partners to provide the very best care.
This content is intended to be educational and not a substitute for professional medical advice, diagnosis, or treatment. Always consult with your physician or other qualified health provider if you have questions regarding a medical condition. Never disregard professional medical advice or delay seeking it as the result of something you have read or seen from BAMF Health.
PLUVICTO® (lutetium Lu 177 vipivotide tetraxetan) is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor pathway inhibition (ARPI) therapy and:
– are considered appropriate to delay taxane-based chemotherapy, or
– have received prior taxane-based chemotherapy
The most common side effects include fatigue, dry mouth, nausea, bone marrow suppression, and renal toxicity.