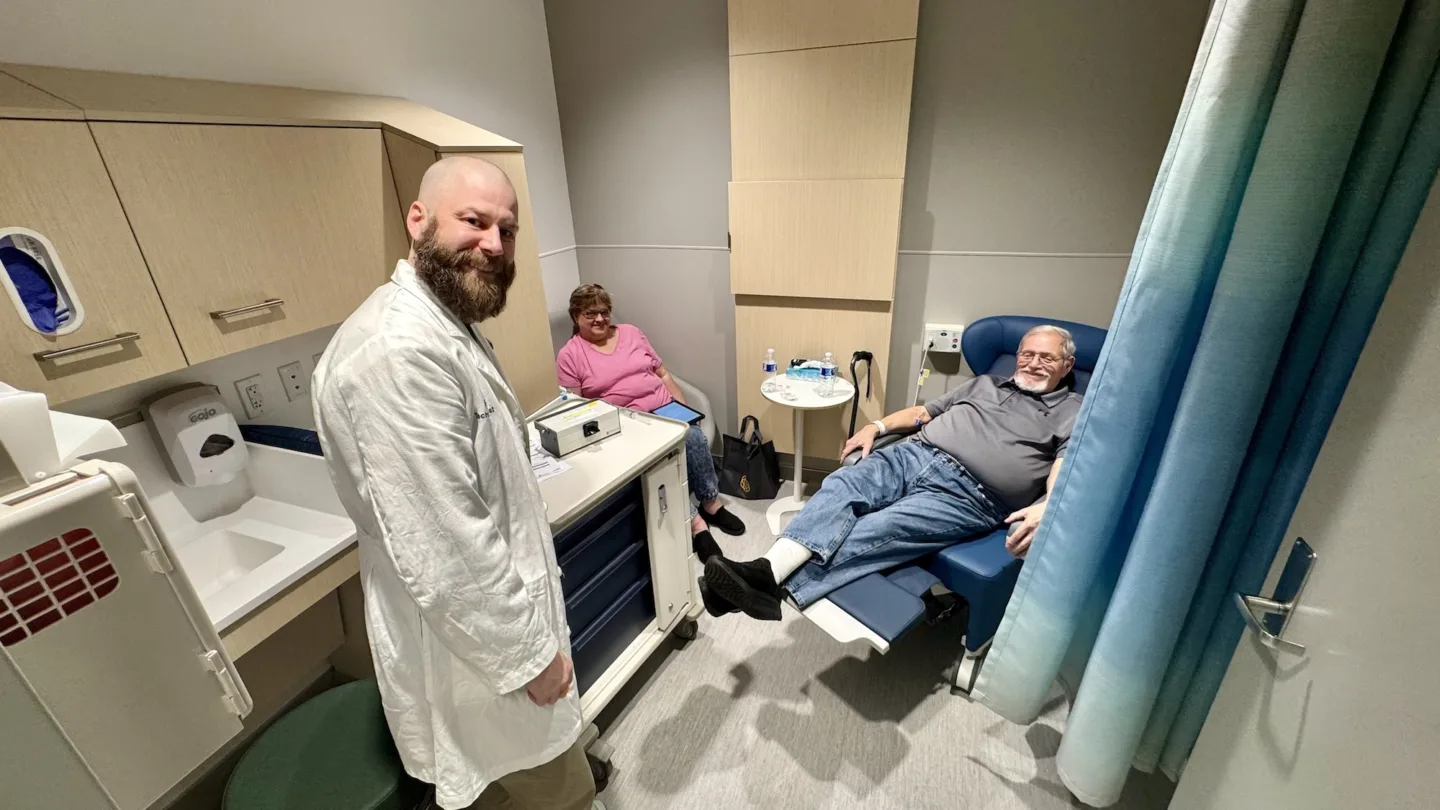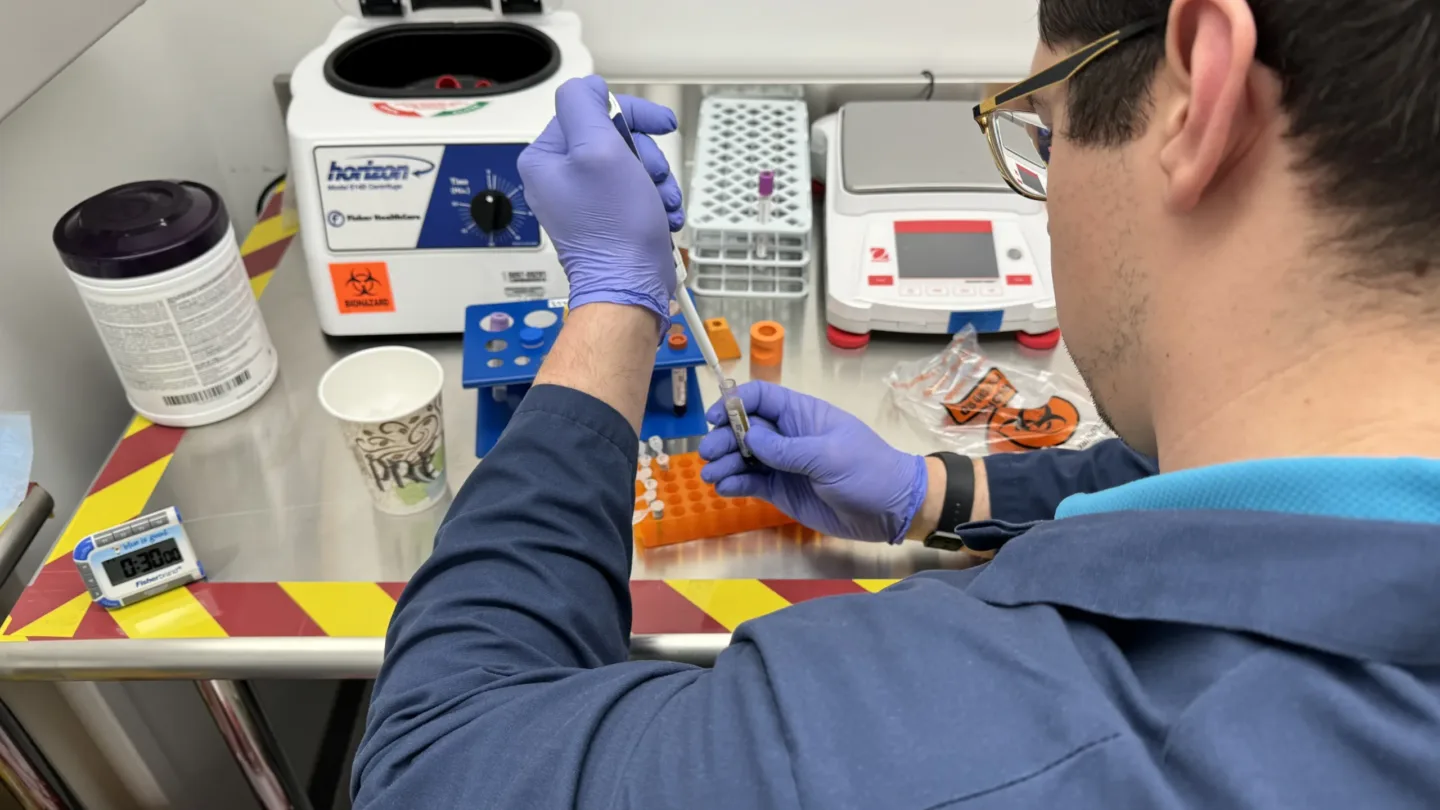
BAMF Health and Nihon Medi-Physics Co., Ltd. (NMP), a leading radiopharmaceutical company in Japan, announced the first patient in the world was imaged at BAMF Health using the novel molecular imaging agent NMK89 developed by NMP as part of a clinical trial. This trial is an important first step in developing improved imaging and a potential therapy for pancreatic cancer.
BAMF Health was selected as one of three sites for this initial clinical trial, and it was the first site to activate. BAMF Health was chosen, in part, because it has the most advanced total-body PET/CT scanner in the world. The exceptional sensitivity and speed of the scanner allow for a more accurate assessment of biodistribution and dosimetry of novel radiopharmaceuticals. Additionally, BAMF Health’s high-efficiency clinical trial platform allows radiopharmaceutical companies to activate trials months faster than traditional sites. In this case, the trial launched just six weeks after NMP selected BAMF Health.
“This milestone represents not just a significant leap forward in our understanding and detection of this challenging disease but also underscores BAMF Health’s commitment to leading the charge in medical innovation,” said Dr. Harshad Kulkarni, MD, Chief Medical Advisor at BAMF Health. “Our efforts today pave the way for a future where pancreatic cancer can be detected with unparalleled precision, offering hope and a new horizon for patients worldwide.”
While pancreatic cancer accounts for only 3% of all cancers in the U.S. and 4% in Japan, it is the deadliest. It is largely discovered in a late stage because symptoms typically don’t arise until it has locally advanced or metastasized. The lack of a targeted molecular imaging agent hampers the ability to detect and monitor pancreatic cancer effectively, highlighting the critical need to improve early detection and guide precision therapy. NMK89, developed by NMP, is an antibody linked to the positron-emitter zirconium-89 (89Zr), that targets a protein called MUC5AC which is overexpressed in pancreatic cancer and several other types of cancer.
This is one of the most logistically complex clinical trials BAMF Health has participated in. It requires multi-disciplinary collaboration between a team of nuclear medicine technologists, radiopharmacists, radiation safety experts, nurses, and clinical trial staff.
The trial also requires significant laboratory capabilities. In order to track how the drug moves through the patient’s body and how much radioactivity is absorbed by the tumors and normal tissue, the team must analyze blood and urine samples at multiple time points from day one to day eight. Additionally, the patient receives multiple PET/CT scans in the same time period. This detailed study helps investigators understand the safety of NMK89, if it can successfully identify pancreatic cancer and its metastases, and the best point in time to visualize those tumors.
“Not only was our nuclear medicine team the first in the world to deliver this promising new radiopharmaceutical, we also had to acquire new skills for processing the trial pharmacokinetics,” said Nuclear Medicine Supervisor Tina Brennan. “The team perfectly coordinated and executed the complex details of this trial and provided excellent patient care at the same time.”
If this trial is successful, NMP and a collaboration partner will develop a same chemical structure complementary alpha (225Ac) therapy (NMT25: usingαSTARTZTM platform technology) to treat pancreatic cancer that expresses the same target, MUC5AC. BAMF Health could be a site for those clinical trials as well.
To view a full list of current and future clinical trials at BAMF Health, visit: www.bamfhealth.com/clinical-trials
About BAMF Health
BAMF Health is the world’s first vertically integrated platform for intelligence-based precision medicine. Headquartered in Grand Rapids, Michigan, BAMF Health employs the most advanced AI-enabled theranostic imaging technology to detect and treat cancer and other diseases and conduct advanced clinical trials. Our overriding mission is to empower patients to become people again. With a team of data scientists, researchers, software engineers, and clinicians —all working in lockstep—we’re making good on it. To learn more about BAMF Health, visit www.bamfhealth.com.
About Nihon Medi-Physics Co., Ltd.
Nihon Medi-Physics (NMP) is a joint venture between Sumitomo Chemical Co., Ltd (Japan) and GE Healthcare (UK). NMP is engaging in ensuring stable supply and research and development of the products as a leading manufacturer of radiopharmaceuticals in Japan. The development of NMK89 is one of the research projects “Development of Antibody Labeling Therapies (with Alpha-Particle) and Companion Diagnostics, in Parallel with Maintenance of Drug Research Facilities to Embody the Concept of Theranostics,” and this research project was adopted by “Cyclic Innovation for Clinical Empowerment (CiCLE)” – FY2017 (2nd Conference) – of the Japan Agency for Medical Research and Development (AMED). This research was supported by AMED under Grant Number JP17pc0101014. “αSTARTZTM” is an abbreviation of “225Ac-α Site Targeting Advanced Radioisotope Treatment via 89Zr-CDx”, which is NMP’s proprietary platform technology for Targeted-Alpha-Therapy. NMP has started to discuss with several Pharma future partnering for αSTARTZ. Official website: https://www.nmp.co.jp/eng/index.html